The Scale to Manage
Complex Global
Clinical Trials
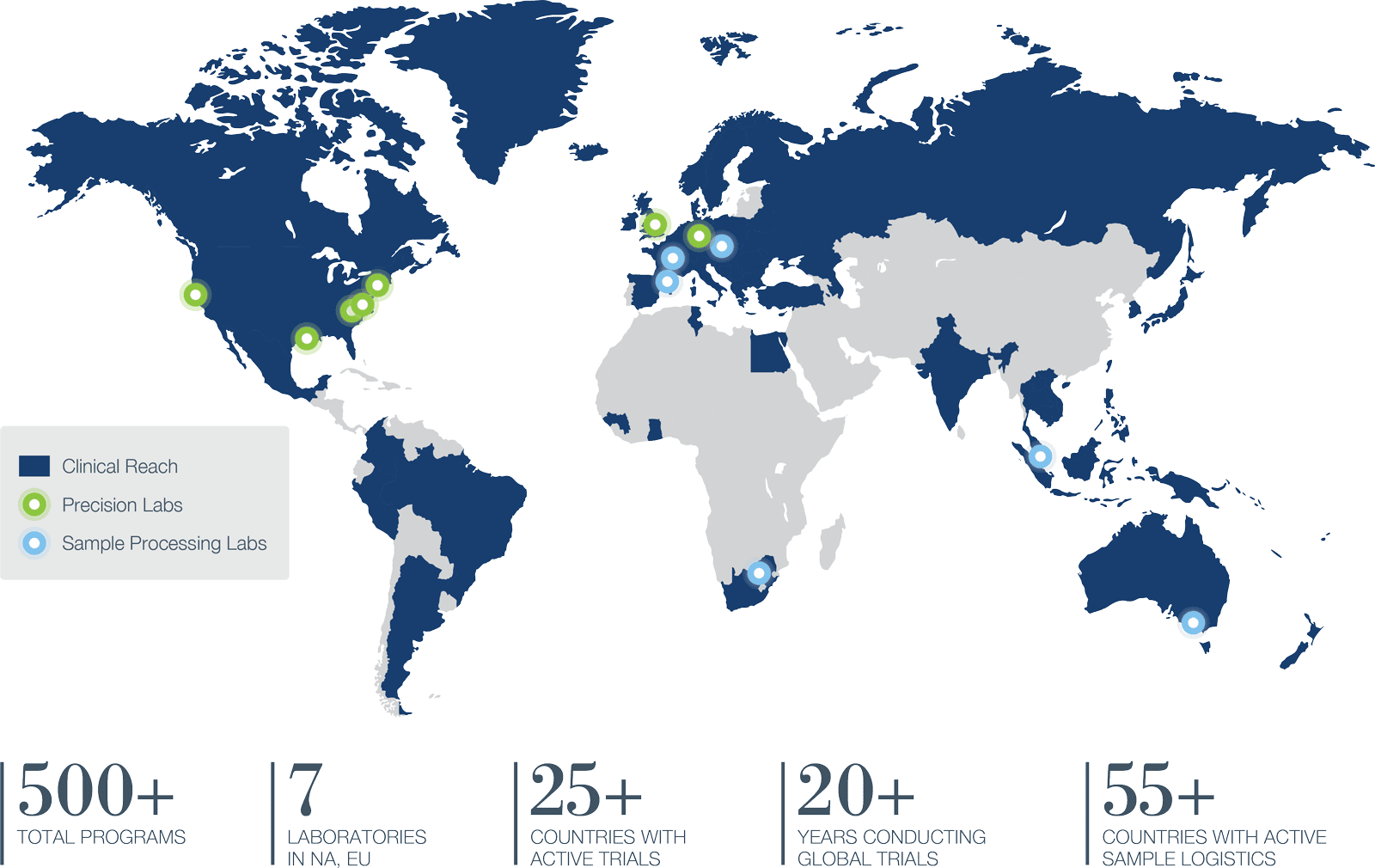
Global clinical trials require consistency from site to site and region to region. Precision supplies it by managing every aspect under a single roof, from strategy and design to full clinical trial execution around the world. We are a leading CRO in the US, Canada, Europe, including Central and Eastern Europe. We also offer APAC CRO services throughout Asia and Australia.
DEDICATED PROFESSIONALS
OFFICES IN NORTH AMERICA, EUROPE AND ASIA/PACIFIC
YEARS CONDUCTING GLOBAL TRIALS
AVERAGE YEARS OF EXPERIENCE BEHIND EVERY CRA
Discover how our Global CRO experts can advance your international clinical trial
Discover how our Global CRO experts can advance your international clinical trial
Related clinical trial services
Clinical Trial Management
We deliver global trial management solutions that leverages the power of biomarkers to optimize trial execution and accelerate your path to approval.
Clinical Development Strategy
We tailor strategies that consider the scientific, regulatory, and commercial factors that will shape your trial to mitigate risk and advance the development pathway.
Clinical Trial Design
We harness advanced trial design approaches—including basket, umbrella, and adaptive trials—to realize the full impact of biomarker-driven clinical research. Deep experience in these highly complex trial designs maximizes both insights and efficiency.
Biostatistics
Our seasoned biostatisticians and statistical programmers deliver insight into every phase of your trial, from study design to regulatory submissions, all backed by meticulous documentation and data monitoring.
Clinical Sample Management
We manage sample inventories across a global network of labs to supply real-time processing in 55 countries, then consolidate data from central labs, screening labs, and specialty labs with clinical data to create actionable reports.
Case Study: Exceeding enrollment expectations in a rescue study: A phase 3 registrational trial in multiple myeloma
Precision was identified by a sponsor to rescue global and US management of a phase 3 registrational trial in relapsed, refractory multiple myeloma patients. This study targeted the enrollment of 780 patients at 155 sites in 20 countries.
The sponsor’s primary motivation for pursuing the shift to Precision from the other contract research organization (CRO) was based on their recognition that clinical research associates (CRAs) with little to no multiple myeloma experience and only minimal monitoring experience had been placed on their study. As a result of inefficiencies in operations, the study’s start-up was delayed.
