Cell and Gene Therapy Resources: Case Studies, White Papers, and More
Novel expertise for novel therapies with Precision ADVANCE’s Cell and Gene Therapy Resources
Overviews
Briefs
Case Studies
White Papers
Publications
Videos
Resources
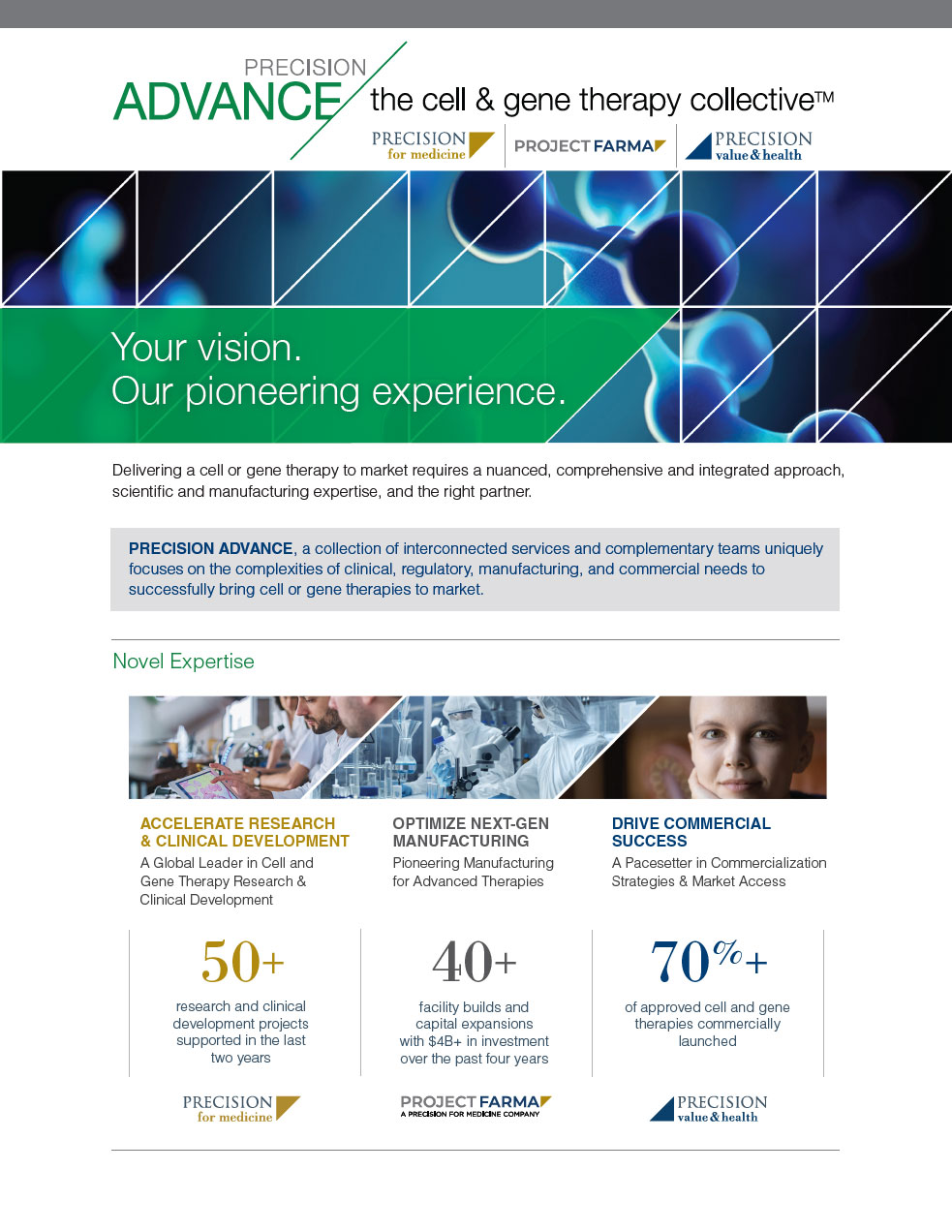
Novel expertise for novel therapies
Case studies
White papers
Publications
Videos
Connect with our thought leaders